Abstract: Patients with cirrhosis who are infected with hepatitis C virus (HCV) are the most in need of antiviral treatment. Virologic cure improves fibrosis and quality of life while reducing liver-related morbidity and mortality. In mid-2011, the addition of direct-acting antiviral agents (DAAs)—the protease inhibitors boceprevir (Victrelis, Merck) and telaprevir (Incivek, Vertex)—to pegylated interferon a-2a/b and ribavirin revolutionized the treatment of HCV infection by increasing cure rates across all fibrosis scores in patients with genotype 1 HCV infection. However, patients with advanced fibrosis or cirrhosis are the most difficult to treat, and the addition of DAAs increases treatment side effects as well as potency. Five phase III DAA trials have been published to date, but they contain limited data on patients with cirrhosis. This review will examine the available data and will describe the evolution of HCV therapy in patients with cirrhosis from the standard-of-care therapy of the past decade into the new era of DAAs.
More than 170 million people, comprising 2–3% of the world’s population, are chronically infected with hepatitis C virus (HCV).1,2 HCV infection is the leading cause of liver-related mortality and the most common indication for liver transplantation (LT) in the United States.1,2 Chronic HCV infection has a prevalence of more than 3 million people in the United States, most of whom are unaware of their disease.3,4 The prognosis of HCV infection varies according to fibrosis progression, with cirrhosis developing in 5–25% of patients over a period of 25–30 years.5,6 Decompensation from HCV-related cirrhosis occurs in 30% of patients over 10 years, with a 3% annual risk of hepatocellular carcinoma (HCC) among cirrhotic patients in North America and Europe.7,8 Mathematical modeling of the natural history of HCV infection projects a peak prevalence of 1 million people with cirrhosis in the United States by 2020 and increasing rates of decompensation and HCC for another 10–13 years.9 Therefore, patients with HCV-related cirrhosis are the most in need of treatment.10,11 Although HCV infection is challenging to treat in this special group, HCV eradication has been demonstrated to improve fibrosis and quality of life while reducing liver-related morbidity and mortality.12-15
The treatment of HCV infection was revolutionized in mid-2011 with the addition of direct-acting antiviral agents (DAAs)—the protease inhibitors boceprevir (Victrelis, Merck) and telaprevir (Incivek, Vertex)—to the decade-long standard-of-care (SOC) therapy of pegylated interferon a-2a/b and ribavirin. This advance resulted in a tremendous demand for HCV therapy, leading to resource rationing and treatment triage.10 The concept of distributive justice with scarce resources suggests that patients with cirrhosis have the greatest need for treatment and thus should receive the highest priority for treatment, with asymptomatic patients with minimal fibrosis being at the other end of the spectrum.10 Our initial experience with DAA therapy reflects this urgency: Of the first
98 consecutive HCV-infected patients we started on telaprevir, almost 40% had advanced fibrosis or cirrhosis.16 This review will examine the data on DAAs in patients with cirrhosis and will describe the evolution of HCV therapy in this special group from the SOC therapy of the past decade into the new era of DAAs.
Why Should Clinicians Treat Patients with Cirrhosis?
The 2004 American Association for the Study of Liver Diseases (AASLD) practice guideline for the diagnosis, management, and treatment of HCV infection recommends treatment for patients with compensated HCV-related cirrhosis who have preserved hepatic synthetic function and sufficient platelet and white blood cell counts to tolerate therapy.17 Achievement of sustained virologic response (SVR) in patients with cirrhosis has been demonstrated to improve liver function and fibrosis, to reduce the incidence of liver-related complications (by ameliorating portal hypertension), to reduce the risk of HCC, and to reduce mortality.18-26 (For a definition of SVR and other commonly used terms, see Table 1.) However, the risk of HCC development is not completely eliminated with achievement of SVR in cirrhotic patients, with the incidence of HCC remaining at 0.6–2.5% in this population.21-23,27 In addition, cirrhosis itself is one of the strongest predictors of treatment failure.19,28 SVR rates are low (10–33%) in patients with genotype 1 HCV infection and compensated cirrhosis who are treated with either interferon a or pegylated interferon a plus ribavirin; higher rates are seen in patients with genotype 2 or 3
HCV infection (33–72%).26,29-37 Despite these disappointing results, a recent decision-analysis model demonstrated that, compared to no treatment, SOC treatment for HCV infection among patients with compensated cirrhosis increased quality-adjusted life-years (QALYs) by 0.950 and saved $55,314; in comparison, improvements were only 0.044 QALYs and $5,511 for patients with decompensated cirrhosis and 0.044 QALYs and $3,223 for patients with post-LT advanced recurrence.38 A similar Markov model with the addition of DAAs further demonstrated a 28% reduction in HCC lifetime risk and an 8% increase in QALYs, compared to SOC therapy.39 Whether DAAs can meet their great expectations in curing patients with HCV-related cirrhosis in a real-world setting remains to be seen; our initial experience with boceprevir and telaprevir has demonstrated increased side effects in addition to increased potency.
The urgency for HCV treatment is potentially at its peak in patients with decompensated cirrhosis, given their lower 5-year survival rate (50%) compared to patients with compensated cirrhosis (91%).6 However, patients with decompensated cirrhosis are the most difficult to treat, with the 3 largest studies of SOC therapy in patients with genotype 1 HCV infection and decompensated cirrhosis demonstrating dismal SVR rates (7–16%).18,40,41 Although LT is the treatment of choice for patients with decompensated cirrhosis, recurrence of HCV infection occurs in 100% of patients, and accelerated post-LT disease is common.17 Thus, clinicians still have a strong incentive to achieve SVR or HCV RNA undetectability prior to LT in order to prevent recurrence, as long as the risks of pre-LT treatment are acceptable.17,35
Hepatitis C Virus Treatment in Patients with Cirrhosis: How Decompensated Is Decompensated?
Compensated cirrhosis has been defined by the presence of preserved liver function (total serum bilirubin <1.5 g/dL,
international normalized ratio <1.5, and albumin
>3.4 g/dL) and the absence of clinical complications such as jaundice, ascites, variceal bleeding, or hepatic encephalopathy.17,19,42 These patients typically have Child-Pugh class A cirrhosis and adequate biochemical indices for possible HCV treatment as defined in the AASLD guideline: neutrophil count above 1.5 k/mm3, platelet count above 75,000 k/mm3, hemoglobin level greater than 13 g/dL for men or greater than 12 g/dL for women, and creatinine level below 1.5 mg/dL.17 The safety and tolerability of SOC treatment, including the rate of discontinuation, do not differ between patients with compensated HCV-related cirrhosis and noncirrhotic patients; however, dose reduction is more frequent in the former group, largely due to hematologic toxicity.19 An oft-cited concern is that treatment in patients with compensated cirrhosis might accelerate hepatic decompensation, as can occur with interferon a therapy in patients with mildly decompensated hepatitis B
virus–related cirrhosis, but the reported rate of decompensation in randomized controlled trials of HCV-infected patients has been negligible (0–3%).32-34,43 This finding is likely related to selection bias and is not necessarily reflective of real-world experience.
In contrast, decompensated cirrhosis is defined as the presence of any of the aforementioned clinical complications; these patients usually have Child-Pugh class B or C cirrhosis.17,19,42 The achievement of SVR is possible in these high-risk patients.18,40-42,44-53 A recent long-term follow-up study by Iacobellis and colleagues of SOC treatment in patients with decompensated cirrhosis confirmed the results of their prior studies: Patients who achieved SVR had a durable reduced rate of decompensation events (33.3% vs 96.1% for non-SVR patients), fewer hospitalizations (7.5 times more likely in non-SVR patients) and reduced mortality (73-month survival for SVR patients vs 53-month survival for non-SVR patients).54 Again, achievement of SVR did not reduce the incidence of HCC.
Treatment with SOC therapy in this population is also associated with increased risks of infection (odds ratio [OR], 2.95; 95% confidence interval [CI], 0.93–9.3) and mortality related to infection (OR, 1.97; 95% CI, 0.40–9.51), especially in the sickest patients with Child-Pugh class C cirrhosis and those with Model for End-Stage Liver Disease scores greater than 18 points.18 This finding is expected due to the reduced blood clearance of endotoxin and bacteria in the acquired immunodeficiency state of cirrhosis.55,56 A subsequent multivariate analysis demonstrated that several factors were associated with SVR: complete early virologic response (cEVR), genotype 2 or 3 HCV infection, and receiving the full duration and dosage of therapy.41 These factors can be used to triage patients early in the course of HCV treatment.
The earliest guideline commenting on HCV treatment in patients with decompensated cirrhosis was from the first International Liver Transplantation Society expert panel consensus conference on LT and HCV infection in 2003. This guideline recommends strongly considering treatment for patients with a Child-Pugh score less than or equal to 7, possibly treating patients with scores of 8–11, and avoiding treatment in patients with scores greater than 11.57 The 2004 AASLD guideline recommends that a low accelerating dose regimen (LADR) be used in patients with “mild degrees of hepatic compromise, as long as treatment is administered by experienced clinicians, with vigilant monitoring for adverse events, preferably in patients who have already been accepted as candidates for [LT].”17 The 2007 Asian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management, and treatment of HCV infection recommend no antiviral treatment for patients with decompensated cirrhosis in the general setting; instead, these patients should be referred for LT.58 The 2011
European Association for the Study of the Liver clinical practice guidelines are more specific: In patients with Child-Pugh class B cirrhosis, “antiviral therapy [can be] offered on an individual basis in experienced centers, preferentially in patients with predictors of good response”; however, patients with Child-Pugh class C cirrhosis should not be treated due to a high risk of life-threatening complications.59
Table 1. Commonly Used Terms in Hepatitis C Virus (HCV) Treatment
| Term | Definition |
| Treatment endpoint | |
| Rapid virologic response (RVR) | HCV RNA levels are undetectable at Treatment Week 4. |
| Extended rapid virologic response (eRVR) | HCV RNA levels are undetectable at Treatment Weeks 4 and 12. |
| Complete early virologic response (cEVR) | HCV RNA levels are detectable at Treatment Week 4 but undetectable at Treatment Week 12. |
| Sustained virologic response (SVR) | HCV RNA levels are undetectable 6 months after the end of treatment. |
| Prior response to failed HCV treatment | |
| Relapse | HCV RNA levels are undetectable at the end of treatment but reappear after the end of treatment. |
| Partial response | HCV RNA levels decline by ≥2 log10 IU/mL but remain detectable at Treatment Week 24. |
| Null response | HCV RNA levels do not decline by ≥2 log10 IU/mL at Treatment Week 12. |
| Breakthrough | After HCV RNA levels have been undetectable, they are detected again during treatment. |
Table 2. Summary of Boceprevir and Telaprevir Phase III Trials
| Trial | Treatment-naïve or treatmentexperienced patients? |
DAA studied | Patients with advanced fibrosis or cirrhosis |
SVR in patients with advanced fibrosis or cirrhosis treated with SOC |
SVR in patients with advanced fibrosis or cirrhosis treated with DAAs and SOC (48 weeks total) |
Overall SVR rate |
| SPRINT-2 | Naïve | Boceprevir | 100/1,097 (9%) | 38% | 52% | 68% |
| ADVANCE | Naïve | Telaprevir | 231/1,088 (21%) |
33% | 62% | 75% |
| ILLUMINATE | Naïve | Telaprevir | 149/540 (28%) | N/A | 63% | 72% |
| HCVRESPOND- 2 |
Experienced | Boceprevir | 78/403 (19%) | R: 2/10 (20%) PR: 0/5 (0%) NR: N/A |
R: 15/18 (83%) PR: 11/22 (50%) NR: 2/4 (50%)* |
R: 77/103 (75%) PR: 30/58 (52%) NR: 16/42 (38%)* |
| REALIZE | Experienced | Telaprevir | 316/663 (48%) | R: 3/30 (10%) PR: 1/10 (10%) NR: 1/19 (5%) |
R: 109/119 (85%) PR: 21/50 (42%) NR: 23/88 (26%) |
R: 245/286 (86%) PR: 55/97 (57%) NR: 46/147 (31%) |
*Subanalysis from the PROVIDE study.93
DAA=direct-acting antiviral agent; N/A=not available; NR=null responders; PR=partial responders; R=relapsers; SOC=standard of care; SVR=sustained virologic response.
Treatment with Direct-Acting Antiviral Agents in Hepatitis C Virus–Related Cirrhosis:
Phase III Trials
Much has been written about the 5 phase III DAA trials that tested the first-generation protease inhibitors boceprevir and telaprevir for the treatment of genotype 1
HCV infection, all of which were recently published in
The New England Journal of Medicine.60-64 Overall, SVR rates were increased by nearly 30% with DAA-containing triple therapy compared to SOC therapy in treatment-naïve patients with genotype 1 HCV infection and by 25–60% in treatment-experienced patients with genotype 1
HCV infection.65
A general discussion of these trials is beyond the scope of this review; instead, this article will focus on the available trial data and subanalyses that assessed use of DAAs in patients with advanced fibrosis and cirrhosis. Note that this special group of patients often represented a small minority of the patients enrolled in these trials, especially in studies of treatment-experienced patients, which makes it difficult to draw conclusions about narrow subsets of patients (eg, prior relapsers with cirrhosis who achieved rapid virologic response [RVR] with boceprevir-based, response-guided therapy [RGT]). Patients who were older than 65 years, infected with genotype 2 or 3 HCV, and/or had decompensated cirrhosis were excluded from these trials. Table 2 summarizes the results of each trial, and Figures 1–5 illustrate the SVR rates achieved in each trial according to fibrosis stage (and prior response, if applicable).
Direct-Acting Antiviral Agent Trials of Treatment-Naïve Patients
SPRINT-2 This trial studied the safety and efficacy of boceprevir-based therapy compared to SOC therapy by comparing 3 treatment regimens: (1) 44 weeks of SOC; (2) 44 weeks of boceprevir plus SOC (fixed-duration therapy [FDT] arm); and (3) a RGT arm in which all patients received boceprevir plus SOC for 24 weeks; those with undetectable HCV RNA levels between Weeks 8 and 24 completed therapy after 24 weeks, while those with detectable HCV RNA levels received SOC therapy for an additional 20 weeks.60 All arms had a 4-week lead-in period with SOC therapy.
About 9% of the patients enrolled in the study (100/1,097) had either advanced fibrosis (Metavir F3; n=47) or cirrhosis (Metavir F4; n=53); these patients had similar baseline characteristics to those with milder fibrosis except for age (mean, 52±8 years vs 49±9 years,
respectively).60,65 The SVR rates in patients with advanced fibrosis or cirrhosis were 52% in the FDT arm, 41% in the RGT arm, and 38% with SOC therapy (Figure 1).66 In addition to other variables, the stage of fibrosis was predictive of SVR, with an SVR rate of 67% for patients with milder fibrosis (Metavir F0–F2) who were treated with boceprevir plus SOC therapy. The relapse rate was also higher in patients with advanced fibrosis or cirrhosis (12–18% vs 9% for patients with milder fibrosis).66 RVR occurred less frequently in patients with advanced fibrosis or cirrhosis (25% vs 46% for patients with milder fibrosis); in this small group (n=33), patients receiving FDT had a higher SVR rate than those receiving RGT: 93% (13/14) versus
79% (11/14), respectively.66 This finding led to the conclusion that genotype 1 HCV–infected, treatment-naïve patients with advanced fibrosis or cirrhosis achieve higher SVR rates with the addition of boceprevir to SOC therapy, but these patients should be treated with FDT: 4 weeks of SOC therapy followed by
44 weeks of boceprevir plus SOC therapy.67
ADVANCE This trial studied the safety and efficacy of telaprevir compared to SOC therapy by exploring 8-week (T8) versus 12-week (T12) courses of telaprevir; the aims of this study were to potentially reduce side effects while preserving efficacy and to evaluate the shortening of therapy to 24 weeks in patients with robust viral responses.61,68 Patients were randomized into 1 of 3 arms: (1) 48 weeks of SOC; (2) T12 plus SOC, followed by SOC for
12 more weeks (if HCV RNA levels were undetectable at Weeks 4 and 12) or 36 more weeks (if HCV RNA levels were detectable at Weeks 4 and 12); or (3) T8 plus SOC, followed by placebo plus SOC for 4 weeks, followed by an additional 12 or 36 weeks of SOC (based on the same HCV RNA criteria described above). Lead-in therapy with SOC was not studied.
Approximately 21% of the patients enrolled in the study (231/1,088) had either advanced fibrosis (n=163) or cirrhosis (n=68), with baseline characteristics in this group similar to those of the overall study population.65 The SVR rates in patients with advanced fibrosis or cirrhosis were 62% in the T12 plus SOC arm, 53% in the T8 plus SOC arm, and 33% with SOC therapy; SVR rates in patients with milder fibrosis were 78%, 74%, and 47%, respectively (Figure 2).61 Overall, patients in the T12 plus SOC group had a higher SVR rate than those in the T8 plus SOC group, with a slightly lower rate of discontinuation of telaprevir in the T12 plus SOC group. Extended RVR (eRVR) was achieved in 58% of patients in the telaprevir arms overall, with over 80% of patients in each arm going on to achieve SVR. Among patients with cirrhosis, 43% (9/21) achieved eRVR; of those patients, 78% (7/9) achieved SVR.68 In conclusion, this study demonstrated that genotype 1 HCV–infected, treatment-naïve patients with advanced fibrosis or cirrhosis achieve higher SVR rates with the addition of telaprevir to SOC therapy, with a reasonable side-effect profile, but these patients should receive a 12-week rather than 8-week course of telaprevir with SOC therapy.
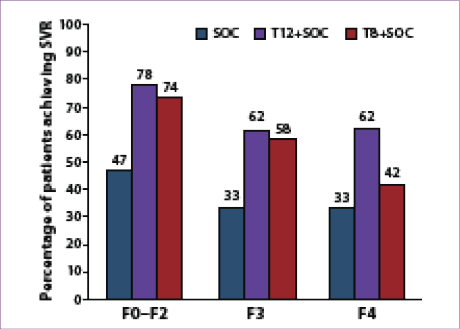
Figure 1. Sustained virologic response (SVR) rates according
to fibrosis stage among boceprevir-treated, treatment-naïve
patients in the SPRINT-2 trial.
FDT=fixed-duration therapy; RGT=response-guided therapy; SOC=standard of care.
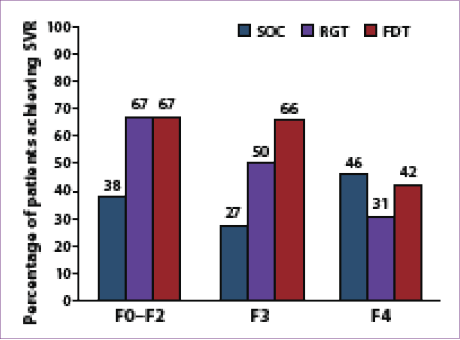
Figure 2. Sustained virologic response (SVR) rates according
to fibrosis stage among telaprevir-treated, treatment-naïve
patients in the ADVANCE trial.
SOC=standard of care; T8+SOC=8 weeks of telaprevir-based triple therapy
followed by SOC for an additional 16 or 40 weeks; T12+SOC=12 weeks of
telaprevir-based triple therapy followed by SOC for an additional 12 or 36 weeks.
ILLUMINATE This open-label, noninferiority trial evaluated the utility of RGT in patients achieving eRVR compared to FDT in patients without eRVR.64 Patients were assigned to 1 of 3 arms: Patients with eRVR while on
T12 plus SOC therapy were randomly assigned to either
(1) 12 more weeks of SOC or (2) 24 more weeks of SOC; (3) patients without eRVR received T12 plus SOC followed by 36 weeks of SOC. There was no SOC control arm.
Approximately 28% of the patients enrolled in the study (149/540) had advanced fibrosis (n=88) or cirrhosis (n=61). The SVR rate was 63% in patients with advanced fibrosis compared to 75% in patients with milder fibrosis (Figure 3). Among patients with advanced fibrosis or cirrhosis who achieved eRVR (46% vs 49%, respectively), there was no statistically significant difference in SVR rates between the RGT and FDT arms (82% vs 88%, respectively).64 However, if the analysis was narrowed to include only patients with cirrhosis, SVR rates were 67% (12/18) in the RGT arm compared to 92% (11/12) in the FDT arm.69 Treatment discontinuation before
Week 20 (n=100) occurred in a higher percentage of patients with advanced fibrosis or cirrhosis (31% vs 19% in patients with milder fibrosis). In conclusion, this study demonstrated that, among genotype 1 HCV–infected, treatment-naïve patients, administering telaprevir as part of a RGT regimen based on eRVR may be appropriate in up to two thirds of patients; RGT should not be used in patients with cirrhosis, given their reduced SVR rate, although a paucity of data are available in this special group.64,68,70,71
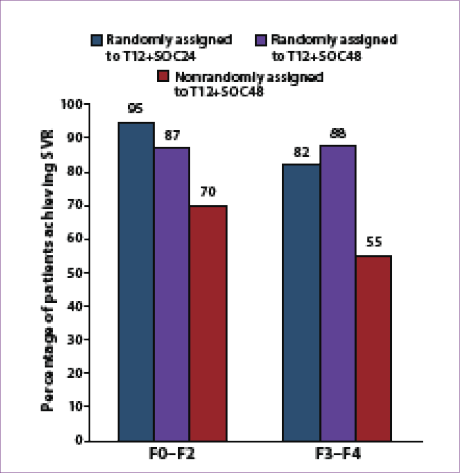
Figure 3. Sustained virologic response (SVR) rates according
to fibrosis stage among telaprevir-treated, treatment-naïve
patients in the ILLUMINATE study.
T12+SOC24=12 weeks of telaprevir plus 24 weeks of standard of care;
T12+SOC48=12 weeks of telaprevir plus 48 weeks of standard of care.
Direct-Acting Antiviral Agent Trials of Treatment-Experienced Patients
HCV-RESPOND-2 This trial enrolled patients who were relapsers or partial responders during prior SOC therapy; patients were randomized to 1 of 4 arms:
(1) RGT consisting of boceprevir plus SOC for 32 weeks if HCV RNA levels were undetectable at Weeks 8 and 12; (2) boceprevir plus SOC for 32 weeks plus an additional 12 weeks of SOC if HCV RNA levels were detectable at Week 8; (3) FDT consisting of boceprevir plus SOC for 44 weeks; or (4) SOC therapy for 44 weeks.62 All arms had a 4-week lead-in with SOC therapy.
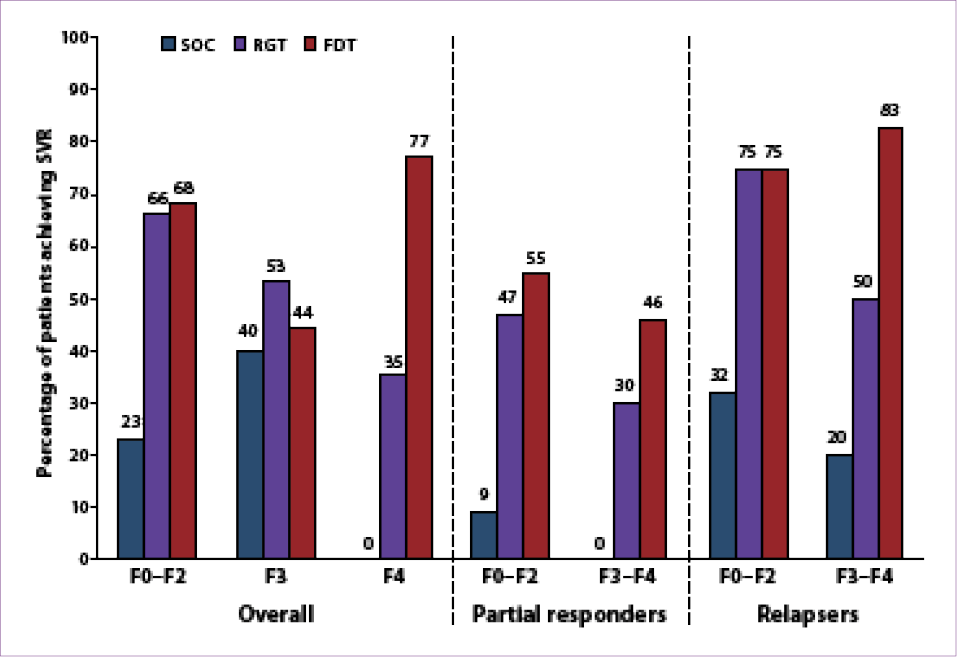
Approximately 19% of the patients enrolled in the study (78/403) had advanced fibrosis (n=29) or cirrhosis (n=49). SVR rates in patients with advanced fibrosis or cirrhosis were 68% in the FDT arm, 44% in the RGT arm, and 13% with SOC therapy; SVR rates in patients with milder fibrosis were 68%, 66%, and 23%, respectively (Figure 4).66 While fibrosis did not affect SVR rates among patients who received FDT, significant fibrosis appeared to have a negative effect on SVR rates among patients who received RGT. Notably, fibrosis did not appear to affect SVR rates in prior relapsers who received FDT (83% in patients with advanced fibrosis or cirrhosis vs 75% in those with milder fibrosis). The relapse rate was higher in patients with advanced fibrosis or cirrhosis (21% vs 11% in patients with milder fibrosis). A decrease of at least 1 log10 IU/mL in
HCV RNA level after the 4-week lead-in period and HCV RNA undetectability at Week 8 were predictive of SVR overall.66 In patients with advanced fibrosis or cirrhosis, achieving both of these benchmarks led to high SVR rates in both the RGT arm (8/10) and the FDT arm (18/20).66 A reduced rate of HCV RNA undetectability at Week 8 (and thus lower rates of SVR) in treatment-experienced, genotype 1 HCV–infected patients with advanced fibrosis or cirrhosis led to the conclusion that FDT with boceprevir, not RGT, optimizes SVR rates compared to SOC therapy.
REALIZE This trial enrolled relapsers, partial responders, and null responders; patients were randomized to 1 of 3 arms: (1) a 4-week lead-in with SOC (as with boceprevir), followed by T12 plus SOC, followed by an additional
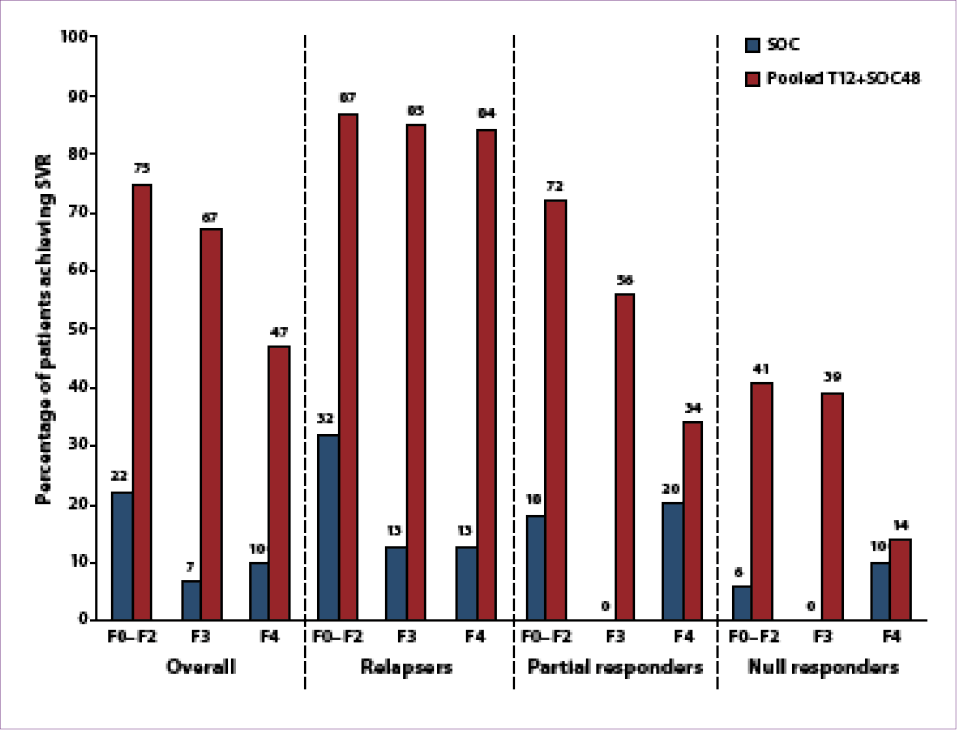
32 weeks of SOC; (2) FDT consisting of T12 plus SOC followed by 36 weeks of SOC; or (3) SOC for 48 weeks.63 In contrast to the studies described above, null responders were enrolled in this study, and nearly half (48%) of the patients enrolled in the study (316/663) had advanced fibrosis (n=147) or cirrhosis (n=169).72 Patients with cirrhosis were slightly older (54 years vs 50 years for noncirrhotic patients), and cirrhotic patients were more likely than noncirrhotic patients to be prior nonresponders
(36% vs 25%, respectively).73 There was no difference in SVR rates between the 4-week lead-in arm and the FDT arm. This study’s higher proportion of patients with cirrhosis allowed for a better evaluation of the difference in treatment outcomes between patients with advanced fibrosis (F3) and those with cirrhosis (F4).
SVR rates were increased in the pooled telaprevir arms compared to SOC therapy although inversely related to the stage of fibrosis: 75% versus 22% in patients with milder fibrosis, 67% versus 7% in patients with advanced fibrosis, and 47% versus 10% in patients with cirrhosis (Figure 5).65,73 As was seen with boceprevir in HCV-RESPOND-2, fibrosis did not appear to affect SVR rates in prior relapsers who received a total of
48 weeks of therapy (84% in patients with cirrhosis vs 87% in those with milder fibrosis).73 The lowest SVR rates occurred in prior null responders, especially those with cirrhosis (14%), although this rate was still higher than the SVR rate achieved with SOC therapy (10%). Relapse rates were higher in patients with cirrhosis and previous partial or null response than in those without cirrhosis (10% vs 4%).73 Rates of discontinuation due to adverse events were also slightly higher in patients with cirrhosis (7% vs 4%). In conclusion, this study confidently demonstrated that genotype 1 HCV–infected, treatment-experienced patients with advanced fibrosis or cirrhosis achieve higher SVR rates with the addition of telaprevir to SOC therapy, but SVR rates in previous null responders with cirrhosis remain low.
Figure 4. Sustained virologic response (SVR) rates according to fibrosis stage and prior response among boceprevir-treated,
treatment-experienced patients in the HCV-RESPOND-2 study.
FDT=fixed-duration therapy; RGT=response-guided therapy; SOC=standard of care.
Safety of Direct-Acting Antiviral Agents in Patients with Cirrhosis
While side effects of HCV therapy are common in all patient types, side effects in patients with cirrhosis tend to be magnified, as these patients often lack the physical and biochemical reserves present in patients with milder fibrosis. Given that nearly all patients with cirrhosis will experience side effects at some point during HCV treatment, providers must become adept troubleshooters. Studies have shown that adverse events occur more frequently with DAAs compared to SOC therapy.50,71
A subanalysis of data from the boceprevir trials demonstrated a similar safety profile (including adverse event–related discontinuation) among patients with advanced fibrosis or cirrhosis and those with milder fibrosis; the exceptions were a slight increase in dose modifications, an increased incidence of anemia (hemoglobin level <10 g/dL), and an increased incidence of dysgeusia among patients with advanced fibrosis or cirrhosis.66 Overall, the incidence of anemia in both trials was greater than 40%, with erythropoetin (EPO) use approaching 40%. Given boceprevir’s longer duration of therapy (24–44 weeks vs 12 weeks with telaprevir), anemia is likely to be greater with boceprevir, although SVR rates in patients managed by ribavirin dose reduction alone were comparable to SVR rates in those managed with EPO.71,74 While the presence of cirrhosis was not a risk factor for on-treatment anemia, on-treatment anemia itself (especially with large hemoglobin declines [>5 g/dL]) was identified as a significant variable for achieving SVR (P<.001).74
A subanalysis of data from the telaprevir trials also demonstrated a similar safety profile among patients with advanced fibrosis or cirrhosis and those with milder fibrosis, except for slight increases in adverse event–related treatment discontinuations (15% vs 11%), rash, anemia, and flu-like symptoms among patients with advanced fibrosis or cirrhosis.73 Because EPO was not allowed in the telaprevir trials, patients with anemia had a 5–6% higher treatment discontinuation rate compared to patients without anemia.71 The overall incidence of anemia approached 40%. Multivariate analysis demonstrated that older age, lower body mass index, more advanced fibrosis, and infection with genotype 1b HCV were significantly associated with the development of on-treatment anemia.75 However, anemia, ribavirin dose reduction, and timing of ribavirin dose reduction did not adversely affect SVR.76 Also, the presence or level of telaprevir resistance was not associated with the stage of fibrosis in patients who failed the telaprevir regimen.65
Figure 5. Sustained virologic response (SVR) rates according to fibrosis stage and prior response among telaprevir-treated,
treatment-experienced patients in the REALIZE study.
SOC=standard of care; T12+SOC48=12 weeks of telaprevir plus 48 weeks of standard of care.
Using Colony-Stimulating Factors with Direct-Acting Antiviral Agents: Real-World Experiences
Although the use of colony-stimulating factors in HCV therapy is not approved by the US Food and Drug Administration and is associated with considerable cost, colony-stimulating factors are commonly and effectively used in clinical practice to initiate, maintain, and improve HCV therapy. Despite the potential adverse events and the black-box warning associated with EPO, practicing clinicians prescribe EPO in 28% of patients treated with SOC therapy.76 This use will likely continue with both boceprevir and telaprevir.77 A recent abstract demonstrated the potential utility of the thrombopoietin-receptor agonist eltrombopag (Promacta, GlaxoSmithKline) as therapy for HCV-related thrombocytopenia; use of this drug permitted the start of antiviral therapy in 95% of patients with platelet counts less than 75,000/mm3 and improved SVR rates with SOC therapy, although at the expense of increased thromboembolic events.78,79 We consider the use of filgrastim (Neupogen, Amgen) when the absolute neutrophil count is below 500/mm3, EPO when the hemoglobin level is below 10 g/dL, and eltrombopag in special circumstances (eg, patients with hemophilia).
In our real-world experience administering telaprevir-based triple therapy in 98 consecutive patients treated at our institution, adverse events were surprisingly common and severe.16 By Week 4, 23% of patients had developed severe anemia (and in many cases required transfusions), 10% had creatinine increases above the upper limit of normal, and 2% discontinued treatment due to severe rash. RVR rates for treatment-naïve patients and relapsers in our case series were significantly lower than those reported in phase III trials (P<.01).
Our initial experience with DAAs is congruent with the results of the French CUPIC study, an early-access cohort study describing real-world experience with boceprevir and telaprevir that includes a large number of treatment-experienced patients with cirrhosis.80 In 455 nonrandomized patients with Child-Pugh class A compensated cirrhosis, rates of serious adverse events (38.4% with boceprevir and 48.6% with telaprevir) and subsequent treatment discontinuation (7.4% and 14.5%, respectively) were higher than those reported in the phase III trials of boceprevir and telaprevir. EPO was used in a majority of patients (66% of patients treated with boceprevir plus SOC and 56.8% of patients treated with telaprevir plus SOC), and blood transfusions were required in 10.7% and 15.2% of patients, respectively.81 Given these findings, clinicians should exercise caution in the real-world use of DAAs in patients with cirrhosis.
This discrepancy in adverse events between real-world settings and the phase III trials of telaprevir and boceprevir likely reflects selection bias and the paucity of data in the subpopulation of patients with cirrhosis. Indeed, the differences between patients enrolled in clinical trials and those treated in real-world settings have been described.82,83 In a study comparing genotype 1
HCV–infected patients enrolled in a DAA trial with those treated with SOC therapy, baseline biochemical profiles were more favorable in study patients, while advanced fibrosis and psychiatric disorders were more common in SOC-treated patients.84 The complexity of DAA therapy, particularly in patients with cirrhosis, requires an organized team approach with healthcare provider expertise and sufficient staff to aid in effective patient education, monitoring, paperwork, and troubleshooting.10 Given the proposed need-based allocation system that prioritizes the sickest patients, as well as the real-world complication rate in patients with cirrhosis, DAA therapy will likely place a strain on healthcare provider time and resources, and this strain may mitigate the achievement of SVR in this subpopulation.
Metabolism of Direct-Acting Antiviral Agents in Patients with Cirrhosis
Boceprevir and telaprevir are extensively metabolized by the liver; boceprevir is metabolized by the aldo-keto reductase system and the cytochrome P450 (CYP) enzyme system, and telaprevir is metabolized solely by the CYP system.70,85 The main route of elimination for both agents is via feces, so there is minimal urinary excretion; thus, dose adjustments are not required in patients with renal insufficiency. Pharmacokinetic studies of boceprevir in patients with Child-Pugh class A, B, and C cirrhosis did not demonstrate any clinically significant differences compared to healthy subjects, so no dose adjustment of boceprevir is required for patients with any degree of cirrhosis; however, boceprevir has not been studied in patients with decompensated cirrhosis nor is it recommended for treatment of this group.85 In contrast, the steady-state exposure to telaprevir was reduced by 46% in HCV-negative patients with Child-Pugh class B cirrhosis compared to healthy subjects, so the appropriate dosing of telaprevir in patients with Child-Pugh class B
and C cirrhosis is not known; thus, treatment with telaprevir is not recommended in these populations.70 Also, patients with cirrhosis are often on many medications with potential drug-drug interactions that can complicate treatment with DAAs. A thorough review of a patient’s medications and the information contained in the package inserts (or an updated website such as
www.hep-druginteractions.org) is recommended so that dose adjustments can be considered prior to initiating DAA therapy.
Treatment with Direct-Acting Antiviral Agents
Before Starting Treatment
For HCV-infected patients with cirrhosis and their doctors, the decision to undergo and continue DAA therapy involves assessment of the risks of possible decompensation and complications versus the likelihood of achieving SVR. In practice, clinicians attempt to achieve a measure of clinical stability (eg, diuresis of ascites, evaluation and prophylaxis against variceal bleeding, and/or reduction of hepatic encephalopathy) and to optimize laboratory indices (eg, starting EPO for baseline anemia) prior to starting DAA therapy in patients with cirrhosis. Also, if patients have Child-Pugh
class B or C cirrhosis or Child-Pugh class A cirrhosis and a history of decompensation, they are referred for LT evaluation prior to starting DAA therapy (as a safety net). Given the limited information on DAAs in patients with cirrhosis, prognosticating the odds of achieving SVR for an individual patient can be very difficult. However, there are some pretreatment and on-treatment variables that can be useful.
Pretreatment Predictors of Sustained Virologic Response in Patients with Cirrhosis
Despite the deluge of information on boceprevir and telaprevir—including study designs, stopping rules, and the effect of prior response to SOC therapy—the foundation of knowledge built over the past decade regarding factors affecting SVR in SOC-treated patients is likely still relevant; these factors include prior response, interleukin (IL)-28B genotype, age, baseline viral load, degree of fibrosis, serum cholesterol level, platelet count, gamma-glutamyl transpeptidase level, alanine aminotrasferase (ALT) level, alcohol intake, and the presence of comorbidities such as insulin resistance.19 Several studies have examined factors predictive of SVR in HCV-infected, SOC-treated patients with both compensated and decompensated cirrhosis, although most of these studies are small case series that predate the discovery of IL-28B. A recent review discusses this topic in detail.19
Another recent review of the phase III DAA trials examines the differences in SVR rates depending on various pretreatment and on-treatment variables.86 Only the REALIZE trial had a sufficient number of patients with cirrhosis to examine pretreatment factors associated with SVR in this group. A recent subanalysis evaluated 117 (69%) of the 169 patients with cirrhosis who had complete pretreatment data.73 Multiple logistic regression analysis demonstrated that baseline levels of low-density lipoprotein and triglycerides; maximum baseline levels of aspartate aminotransferase (AST) and ALT; prior response to SOC therapy; and genotype 1b
HCV infection were significantly associated with achievement of eRVR. However, only high baseline AST and ALT levels and prior relapse following SOC therapy remained significant for predicting SVR among patients with cirrhosis who received T12 plus SOC for 48 weeks.73 It is interesting that genotype 1b HCV infection (compared to genotype 1a) did not remain a statistically significant predictor of SVR because of the increased SVR rates in treatment-naïve patients infected with genotype 1b versus 1a; this finding was demonstrated with both boceprevir (70% vs 63%, respectively) and telaprevir (79% vs 71%, respectively). In the REALIZE trial of telaprevir, genotype 1 subtype did not affect SVR in prior relapsers, whereas genotype 1b
was associated with higher SVR rates than genotype 1a among partial responders (68% vs 47%, respectively) and null responders (37% vs 27%, respectively).72 Given the paucity of data regarding the effect of IL-28B on SVR rates in the phase III DAA trials, the history of prior response to SOC therapy continues to be a crucial pretreatment predictor of SVR in patients with cirrhosis. Thus, specific details of prior HCV treatment should be pursued aggressively and examined carefully prior to initiating DAA therapy.
The discovery of IL-28B dramatically changed our understanding of the likelihood of achieving SVR with SOC therapy in both acute and chronic HCV infection.87,88 A subanalysis of the HALT-C trial demonstrated the importance of several pretreatment variables: IL-28B rs12979860-CC genotype plus 4 clinical variables (low baseline HCV RNA level, low AST/ALT ratio, Ishak fibrosis score of 3 vs 4, and prior exposure to ribavirin) were highly predictive of SVR in patients treated with SOC therapy (without a DAA), with an area under the curve of 78.5%.89 IL-28B genotype is the strongest pretreatment predictor of SVR in genotype 1
HCV–infected patients who are treated with SOC therapy alone or SOC plus either boceprevir or telaprevir using either FDT or RGT.90 In incomplete data sets from SPRINT-2 and ADVANCE, SVR rates in white patients were 80–90% among those with IL-28B genotype CC, approximately 71% among those with the
CT genotype, and 52–59% among those with the
TT genotype.72 The updated AASLD guideline acknowledges the predictive capabilities of IL-28B genotype, but it posits that data are insufficient to support restricting DAA therapy for only CT/TT genotypes because RGT may be additionally beneficial with DAAs for patients with the CC genotype.71,91,92 For example, knowing they are IL-28B genotype CC may be useful for patients with cirrhosis who are borderline candidates for treatment or are unsure about starting HCV treatment.
On-Treatment Predictors of Sustained Virologic Response
A recent meta-analysis of 3 large, randomized,
phase III trials using SOC therapy demonstrated that, on multiple logistic regression analysis, RVR, cEVR, and cumulative ribavirin dose were significantly associated with SVR in genotype 1 HCV–infected patients with advanced fibrosis or cirrhosis.26 It is noteworthy that only on-treatment responses, not pretreatment variables, were significant. The stopping rules were carefully devised for each DAA after analyses of viral kinetics and the likelihood of SVR at specified time points; thus, these rules are the best current guide to predict on-treatment SVR. RVR and eRVR were predictive of SVR in all phase III DAA studies, but these benchmarks were achieved less frequently in patients with cirrhosis. The ILLUMINATE trial of telaprevir suggested a difference in SVR rates based on the presence of cirrhosis among patients who received RGT based on eRVR; specifically, patients with advanced fibrosis had a higher SVR rate than patients with cirrhosis. In patients with cirrhosis, almost half (30/61) achieved eRVR, including 18 patients randomized to RGT and 12 patients randomized to FDT (T12 plus SOC for 48 weeks). The SVR rates were 67% (12/18) for the RGT group compared to 92% (11/12) for the FDT group. Although limited by small numbers, this analysis shows that, even in patients with optimal early responses to DAAs, RGT is inadequate and 48 weeks of treatment is crucial.
One potential strategy to mitigate early side effects in patients with cirrhosis is to utilize a SOC lead-in with a LADR to ease patients into treatment prior to starting either DAA. In this treatment strategy, a decrease greater than 1 log10 IU/mL in HCV RNA level at Week 4 plus HCV RNA undetectability at Week 8 were predictive of SVR in patients with cirrhosis who were treated with boceprevir.93 In contrast, a subanalysis of the REALIZE trial of telaprevir demonstrated that a Week 4 lead-in response did not provide additional guidance over prior response to SOC therapy in the prediction of SVR (in the entire cohort), except in patients for whom data on prior response to SOC therapy are not available and in null responders for whom a decrease in HCV RNA level greater than 1 log10 IU/mL at
Week 4 was associated with a higher SVR rate: 54% (15/28) compared to 15% (6/41) among patients with a less–than–1 log10 IU/mL decrease.94
Are Any Patients with Cirrhosis Eligible for 24 Weeks of Treatment?
Pooling the 5 phase III DAA trials together, 23% of patients had advanced fibrosis or cirrhosis (874/3,791), but only 10.6% had cirrhosis (400/3,791), with patients in REALIZE comprising 42% of the latter group. Thus, it is clear that phase III data on treatment of patients with cirrhosis are lacking, especially regarding RGT. The
phase III trials of treatment-experienced patients demonstrated that fibrosis did not affect SVR rates in prior relapsers who received 48 weeks of total therapy, but RGT was not assessed in these easiest-to-treat patients. At the other end of the spectrum, it appears that prior null responders with cirrhosis not only do not qualify for RGT, but, given their low SVR rates even when treated with DAAs (14–38%), they may not even be candidates for treatment. Specifically, the low SVR rates in this group of toughest-to-treat patients raise significant questions about whether to treat now or wait and whether therapy is cost-effective.
Conclusions
HCV infection is a global health epidemic that can be mitigated by effective treatment and cure. Patients with HCV-related cirrhosis are both the most in need of treatment and the most challenging to treat, but achievement of SVR in this group can improve fibrosis and reduce liver-related morbidity and mortality. In all phase III DAA trials that have been published to date, the presence of cirrhosis was negatively associated with the achievement of SVR. The addition of either boceprevir or telaprevir consistently demonstrated improved SVR rates over SOC therapy alone in patients with cirrhosis, regardless of the experimental treatment arm, and safety profiles in patients with cirrhosis were comparable to those seen in patients with milder fibrosis. Careful and thorough pretreatment evaluation of baseline characteristics, including prior treatment response, and understanding of side-effect management and on-treatment responses with DAA therapy in patients with cirrhosis are crucial to SVR estimation and treatment completion. Clinicians should not lose sight of the ultimate goal of treatment, virologic cure, despite the greater obstacles encountered in patients with cirrhosis.
References
1. Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(suppl 1):
74-81.
2. Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567.
3. Edlin BR. Perspective: test and treat this silent killer. Nature. 2011;474:S18-S19.
4. Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36(suppl):S30-S34.
5. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(suppl 1):
S35-S46.
6. Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296-305.
7. Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472.
8. Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383-398.
9. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-521.
10. Aronsohn A, Jensen D. Distributive justice and the arrival of direct-acting antivirals: who should be first in line? Hepatology. 2011;53:1789-1791.
11. Forns X, Bruix J. Treating hepatitis C in patients with cirrhosis: the effort is worth it. J Hepatol. 2010;52:624-626.
12. Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313.
13. John-Baptiste AA, Tomlinson G, Hsu PC, et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am J Gastroenterol. 2009;104:2439-2448.
14. Bruno S, Crosignani A, Facciotto C, et al. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology. 2010;51:2069-2076.
15. Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280-288.
16. Martel-Laferriere V, Bichoupan K, Pappas A, et al. Effectiveness of HCV triple therapy with telaprevir in New York City. Presented at the 47th Annual Meeting of the European Association for the Study of the Liver; April 18–22, 2012; Barcelona, Spain. Abstract 1137.
17. Strader DB, Wright T, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147-1171.
18. Iacobellis A, Siciliano M, Perri F, et al. Peginterferon alfa-2b and ribavirin in patients with hepatitis C virus and decompensated cirrhosis: a controlled study. J Hepatol. 2007;46:206-212.
19. Vezali E, Aghemo A, Colombo M. A review of the treatment of chronic hepatitis C virus infection in cirrhosis. Clin Ther. 2010;32:2117-2138.
20. Bruno S, Stroffolini T, Colombo M, et al; Italian Association of the Study of the Liver Disease (AISF). Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579-587.
21. Shiratori Y, Ito Y, Yokosuka O, et al; Tokyo-Chiba Hepatitis Research Group. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;142:105-114.
22. Hung CH, Lee CM, Lu SN, et al. Long-term effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. J Viral Hepat. 2006;13:409-414.
23. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677-684.
24. Floreani A, Baldo V, Rizzotto ER, Carderi I, Baldovin T, Minola E. Pegylated interferon alpha-2b plus ribavirin for naive patients with HCV-related cirrhosis. J Clin Gastroenterol. 2008;42:734-737.
25. Cardoso AC, Moucari R, Figueiredo-Mendes C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652-657.
26. Bruno S, Shiffman ML, Roberts SK, et al. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology. 2010;51:388-397.
27. Kobayashi S, Takeda T, Enomoto M, et al. Development of hepatocellular carcinoma in patients with chronic hepatitis C who had a sustained virological response to interferon therapy: a multicenter, retrospective cohort study of 1124 patients. Liver Int. 2007;27:186-191.
28. Afdhal N, Jacobson I, Brown R, et al; The Win-R Study Group. The effect of liver fibrosis and cirrhosis on SVR in 4,913 patients with hepatitis C: results from the Win-R trial. Gastroenterology. 2006;130(4 suppl 2):A-771. Abstract 655.
29. Heathcote EJ, Shiffman ML, Cooksley WG, et al. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med. 2000;343:1673-1680.
30. Helbling B, Jochum W, Stamenic I, et al; Swiss Association for the Study of the Liver (SASL). HCV-related advanced fibrosis/cirrhosis: randomized controlled trial of pegylated interferon alpha-2a and ribavirin. J Viral Hepat. 2006;13:762-769.
31. Abergel A, Hezode C, Leroy V, et al; French multicenter study group. Peginterferon alpha-2b plus ribavirin for treatment of chronic hepatitis C with severe fibrosis: a multicentre randomized controlled trial comparing two doses of peginterferon alpha-2b. J Viral Hepat. 2006;13:811-820.
32. Di Marco V, Almasio PL, Ferraro D, et al. Peg-interferon alone or combined with ribavirin in HCV cirrhosis with portal hypertension: a randomized controlled trial. J Hepatol. 2007;47:484-491.
33. Roffi L, Colloredo G, Pioltelli P, et al; Gruppo Epatologico Lombardo. Pegylated interferon-alpha2b plus ribavirin: an efficacious and well-tolerated treatment regimen for patients with hepatitis C virus related histologically proven cirrhosis. Antivir Ther. 2008;13:663-673.
34. Giannini EG, Basso M, Savarino V, Picciotto A. Predictive value of on-treatment response during full-dose antiviral therapy of patients with hepatitis C virus cirrhosis and portal hypertension. J Intern Med. 2009;266:537-546.
35. Aghemo A, Rumi MG, Monico S, et al. The pattern of pegylated interferon-alpha2b and ribavirin treatment failure in cirrhotic patients depends on hepatitis C virus genotype. Antivir Ther. 2009;14:577-584.
36. Rumi MG, Aghemo A, Prati GM, et al. Randomized study of peginterferon- alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterology. 2010;138:108-115.
37. Cheng WS, Roberts SK, McCaughan G, et al; CHARIOT Study Group. Low virological response and high relapse rates in hepatitis C genotype 1 patients with advanced fibrosis despite adequate therapeutic dosing. J Hepatol. 2010;53:616-623.
38. Saab S, Hunt DR, Stone MA, McClune A, Tong MJ. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: a decision analysis model. Liver Transpl. 2010;16:748-759.
39. Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156:279-290.
40. Everson GT, Trotter J, Forman L, et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255-262.
41. Iacobellis A, Siciliano M, Annicchiarioco BE, et al. Sustained virological responses following standard antiviral therapy in decompensated HCV infected cirrhotic patients. Aliment Pharmacol Ther. 2009;30:146-153.
42. Iacobellis A, Ippolito A, Andriulli A. Antiviral therapy in hepatitis C virus cirrhotic patients in compensated and decompensated condition. World J Gastroenterol. 2008;14:6467-6472.
43. Hoofnagle JH, Di Bisceglie AM, Waggoner JG, Park Y. Interferon alfa for patients with clinically apparent cirrhosis due to chronic hepatitis B. Gastroenterology. 1993;104:1116-1121.
44. Hadziyannis SJ, Sette H Jr, Morgan TR, et al; PEGASYS International Study Group. Peginterferon alpha 2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355.
45. Mangia A, Santoro R, Minerva N, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352:2609-2617.
46. Jensen DM, Morgan TR, Marcellin P, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology. 2006;43:954-960.
47. Ferenci P, Laferl H, Scherzer TM, et al; Austrian Hepatitis Study Group. Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology. 2008;135:451-458.
48. Prati GM, Rumi M, Aghemo A, D’Ambrosio R, De Nicola S, Colombo M. The influence of liver fibrosis on the outcome of pegylated interferon and ribavirin anti-HCV therapy: a sub-analysis of the MIST study. Hepatology. 2009;50(suppl 4):
687A. Abstract 818.
49. Forns X, Garcia-Retortillo M, Serrano T, et al. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39:389-396.
50. Annicchiarico BE, Siciliano M, Avolio AW, et al. Treatment of chronic hepatitis C virus infection with pegylated interferon and ribavirin in cirrhotic patients awaiting liver transplantation. Transplant Proc. 2008;40:1918-1920.
51. Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl. 2002;8:350-355.
52. Tekin F, Gunsar F, Karasu Z, Akarca U, Ersoz G. Safety, tolerability, and efficacy of pegylated-interferon alfa-2a plus ribavirin in HCV-related decompensated cirrhotics. Aliment Pharmacol Ther. 2008;27:1081-1085.
53. Carrion JA, Martinez-Bauer E, Crespo G, et al. Antiviral therapy increases the risk of bacterial infections in HCV-infected cirrhotic patients awaiting liver transplantation: a retrospective study. J Hepatol. 2009;50:719-728.
54. Iacobellis A, Perri F, Valvano MR, Caruso N, Niro GA, Andriulli A. Long-term outcome after antiviral therapy of patients with hepatitis C virus infection and decompensated cirrhosis. Clin Gastroenterol Hepatol. 2011;9:249-253.
55. Jacob AI, Goldberg PK, Bloom N, Degenshein GA, Kozinn PJ. Endotoxin and bacteria in portal blood. Gastroenterology. 1977;72:1268-1270.
56. Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727-738.
57. Wiesner RH, Sorrell M, Villamil F; International Liver Transplantation Society Expert Panel. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9:S1-S9.
58. Asian Pacific Association for the Study of the Liver (APASL) Hepatitis C Working Party, McCaughan GW, Omata M, et al. Asian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management and treatment of hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:615-633.
59. Calvaruso V, Craxì A. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int. 2012;32(suppl 1):2-8.
60. Poordad F, McCone J Jr, Bacon BR, et al; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206.
61. Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416.
62. Bacon BR, Gordon SC, Lawitz E, et al; HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217.
63. Zeuzem S, Andreone P, Pol S, et al; REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428.
64. Sherman KE, Flamm SL, Afdhal NH, et al; ILLUMINATE Study Team. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024.
65. Bourlière M, Khaloun A, Wartelle-Bladou C, et al. Future treatment of patients with HCV cirrhosis. Liver Int. 2012;32(suppl 1):113-119.
66. Bruno S, Vierling JM, Esteban R, et al. Boceprevir in addition to standard of care enhanced SVR in hepatitis C virus (HCV) genotype-1 with advanced fibrosis/cirrhosis: subgroup analysis of SPRINT-2 and RESPOND-2 studies. J Hepatol. 2011;54(suppl 1):S4.
67. Manns MP, Markova AA, Serrano BC, Cornberg M. Phase III results of boceprevir in treatment naïve patients with chronic hepatitis C genotype 1. Liver Int. 2012;32(suppl 1):27-31.
68. Liapakis AM, Jacobson I. Telaprevir user’s guide. Liver Int. 2012;32(suppl 1):
17-25.
69. Marcellin P, Sullivan JC, Fried MW, et al. Sustained virologic response rates and viral resistance profiles were similar in patients treated with a telaprevir-based regimen regardless of liver fibrosis stage. Presented at the 62nd Annual Meeting of the American Association for the Study of Liver Diseases; November 4–8, 2011; San Francisco, California. Abstract 2105.
70. Incivek (telaprevir) [package insert]. Cambridge, Mass: Vertex Pharmaceuticals; 2012.
71. Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB; American Association for Study of Liver Diseases. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433-1444.
72. Zeuzem S, Andreone P, Pol S, et al. REALIZE trial final results: telaprevir-based regimen for genotype 1 hepatitis C virus infection in patients with prior null response, partial response or relapse to peginterferon/ribavirin. Presented at the 46th Annual Meeting of the European Association for the Study of the Liver; March 30–April 3, 2011; Berlin, Germany. Abstract 5.
73. Pol S, Roberts SK, Andreone P, et al. Efficacy and safety of telaprevir-based regimens in cirrhotic patients with HCV genotype 1 and prior peginterferon/ribavirin treatment failure: subanalysis of the REALIZE phase III study. Hepatology. 2011;54(suppl):374A-375A. Abstract 31.
74. Sulkowski MS, Poordad F, Manns MP, et al. Anemia during treatment with peginterferon alfa-2b/ribavirin with or without boceprevir is associated with higher SVR rates: analysis of previously untreated and previous-treatment-failure patients. J Hepatol. 2011;54(suppl 1):S194-S195. Abstract 476.
75. Roberts SK, Andreone P, Pol S. Impact of anemia and ribavirin dose reduction on SVR to a telaprevir-based regimen in patients with HCV genotype 1 and prior peginterferon/ribavirin treatment failure in the phase III REALIZE study. Hepatology. 2011;54(suppl):1007A-1008A. Abstract 1368.
76. Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.
77. Limaye AR, Draganov PV, Cabrera R. Boceprevir for chronic HCV genotype 1
infection. N Engl J Med. 2011;365:176; author reply 177-178.
78. McHutchison JG, Dusheiko G, Shiffman ML, et al; TPL102357 Study Group. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227-2236.
79. Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007.
80. Hézode C, Dorival C, Zoulim F, et al. Safety of telaprevir or boceprevir in combination with peginterferon alfa/ribavirin, in cirrhotic non responders. First results of the French early access program (ANRS CO20-CUPIC) in real-life setting. Presented at the 69th Annual Meeting of the French Association for the Study of the Liver; September 28–October 1, 2011; Paris France.
81. Hezode C, Dorival C, Zoulim F, et al. Safety of telaprevir or boceprevir in combination with peginterferon alfa/ribavirin, in cirrhotic nonresponders. First results of the French early access program (ANRS CO20-CUPIC). Presented at the 47th Annual Meeting of the European Association for the Study of the Liver; April 18–22, 2012; Barcelona, Spain. Abstract 8.
82. Davidson MH. Differences between clinical trial efficacy and real-world effectiveness. Am J Manag Care. 2006;12(15 suppl):S405-S411.
83. Martin K, Bégaud B, Latry P, Miremont-Salamé G, Fourrier A, Moore N. Differences between clinical trials and postmarketing use. Br J Clin Pharmacol. 2004;57:86-92.
84. Beinhardt S, Staettermayer AF, Rutter K, et al. Treatment of chronic hepatitis C genotype 1 patients at an academic center in Europe involved in prospective, controlled trials: is there a selection bias? Hepatology. 2012;55:30-38.
85. Victrelis (boceprevir) [package insert]. Whitehouse Station, NJ: Merck Pharmaceuticals; 2012.
86. Kwo PY. Phase III results in genotype 1 naïve patients: predictors of response with boceprevir and telaprevir combined with pegylated interferon and ribavirin. Liver Int. 2012;32(suppl 1):39-43.
87. Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28b predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401.
88. Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28b and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801.
89. O’Brien TR, Everhart JE, Morgan TR, et al; HALT-C Trial Group. An IL28B genotype-based clinical prediction model for treatment of chronic hepatitis C. PLoS One. 2011;6:e20904.
90. Akuta N, Suzuki F, Hirakawa M, et al. Amino acid substitution in hepatitis C virus core region and genetic variation near the interleukin 28B gene predict viral response to telaprevir with peginterferon and ribavirin. Hepatology. 2010;52:421-429.
91. Poordad F, Bronowicki JP, Gordon SC, et al. IL28B polymorphism predicts virologic response in patients with chronic hepatitis C genotype 1 treated with boceprevir (BOC) combination therapy. J Hepatol. 2011;54(suppl 1):S6. Abstract 12.
92. Jacobson IM, Catlett I, Marcellin P, et al. Telaprevir substantially improves SVR rates across all IL28b genotypes in the ADVANCE study. J Hepatol. 2011;54(suppl 1):S542-S543. Abstract 1369.
93. Vierling JM, Flamm SL, Gordon SC, et al. Efficacy of boceprevir in prior non-responders to peginterferon/ribavirin: the PROVIDE study. Presented at the 62nd Annual Meeting of the American Association for the Study of Liver Diseases; November 4–8, 2011; San Francisco, California. Abstract 931.
94. Zeuzem S, Foster GR, Andreone P, et al. Different likelihood of achieving SVR on a telaprevir-containing regimen among null responders, partial responders and relapsers irrespective of similar responses after a peginterferon/ribavirin 4-week lead-in phase: REALIZE study subanalysis. Hepatology. 2011;54(suppl):986A-987A. Abstract 1331.