Gastric Phytobezoar Dissolution with Ingestion of Diet Coke and Cellulase
Scott J. Kramer, MD1
Mark B. Pochapin, MD2
1Department of Medicine, Weill Cornell Medical College and New York-Presbyterian Hospital, New York, New York; 2Jay Monahan Center for Gastrointestinal Health, Division of Gastroenterology and Hepatology, Weill Cornell Medical College and New York-Presbyterian Hospital, New York, New York
Address correspondence to:
Dr. Mark B. Pochapin, NYU Langone Medical Center, 522 First Avenue,
Suite 901B, New York, NY 10016; Tel: 646-501-6760; Fax: 646-501-6767; E-mail: Mark.Pochapin@nyumc.org
A bezoar is an indigestible mass of material—such as hair, food, seeds, or another ingested substance—found in the gastrointestinal tract.1 A phytobezoar, the most common type of bezoar, is composed of indigestible fruit and vegetable fibers, such as cellulose, hemicellulose, lignin, or tannins. Most phytobezoars occur in patients who have impaired gastric motility or digestion, usually following gastric surgery (such as a Billroth I or II gastrectomy) or as a consequence of impaired motility in patients with diabetic gastroparesis, mixed connective tissue disease, or hypothyroidism.2,3 Impaired gastric peristalsis, low gastric acidity, and loss of normal pyloric function can all contribute to phytobezoar formation.4 Patients with phytobezoars may experience epigastric pain or discomfort, nausea, vomiting, early satiety, weight loss, diarrhea, dysphagia, or upper gastrointestinal ulcerations and hemorrhage.1,2
Prior to the 1960s, phytobezoars were often treated with surgery; however, a wide variety of therapeutic options have since been reported, including medical treatment with cellulase, papain, metoclopramide, or N-acetylcysteine.1,2,4-6 Surgical intervention is still sometimes necessary, and endoscopic removal has also been used.7-9 Recently, the carbonated beverage Coca-Cola (TheCoca-Cola Company) has been successfully used to dissolve bezoars.3 We report 3 patients with gastric phytobezoars who were successfully treated with a combination of Diet Coke (The Coca-Cola Company) and cellulase.
Case Series
Over the course of several months, 3 patients presented to our institution with endoscopically documented phytobezoars. These patients included 2 men and 1 woman, and all of the patients had 1 or more factors that predisposed them to bezoar formation. Patient #1 was a 68-year-old man with diabetic gastroparesis who had undergone an esophagectomy with gastric pull-up for treatment of esophageal cancer. He presented with postprandial bloating, nausea, and vomiting of several months’ duration. Patient #2 was a 76-year-old diabetic man who presented with postprandial dysphagia and belching. Patient #3 was an 83-year-old woman who had undergone a Billroth I gastrectomy 45 years earlier for treatment of peptic ulcer disease. This patient presented with a 2-month history of intermittent postprandial nausea and vomiting, as well as decreased appetite and weight loss. All of these patients were referred for treatment of the symptoms described above and were diagnosed with a phytobezoar during an esophagogastroduodenoscopy. At the time of diagnosis, no attempt was made to remove or mechanically disrupt the bezoar.
After diagnosis of the phytobezoar, each patient was instructed to drink one 12-oz can of Diet Coke twice daily. Additionally, each patient started treatment with cellulase. As cellulase is not readily available by prescription in US pharmacies, the patients were told to visit a local health food store to find the enzymatic supplement that contained the largest amount of cellulase. Cellulase is only available in combination with other enzymes, and the amount of cellulase in each tablet varies depending on the manufacturer. The patients were told to take 1 cellulase tablet twice daily in addition to the Diet Coke. Patient #1 also received 5 injections (of 20 units each) of onabotulinumtoxin A (Botox, Allergan) to the pylorus for treatment of gastroparesis. All of the patients were treated until resolution of the phytobezoar could be documented via endoscopy (Figure 1); therefore, the treatment time varied among the patients. The interval of time from the initiation of treatment to endoscopic confirmation of bezoar resolution was 8 weeks for Patient #1, 6 weeks for Patient #2, and 8 weeks for Patient #3 (Table 1).
Since anecdotal reports had previously suggested the possible efficacy of Coca-Cola and cellulase for treatment of bezoars and there is no known significant toxicity for either of these agents, institutional review board approval was deemed to be unnecessary.
Discussion
In the past, phytobezoars have been medically treated using cellulase (an enzyme that digests cellulose) or papain (a proteolytic enzyme currently found in Adolph’s Meat Tenderizer [Lawry’s]).4-6 Other medical agents that have been used include metoclopramide, N-acetylcysteine, and combination therapy consisting of cellulase, cysteine, and metoclopramide.1,2,10 Surgical removal of phytobezoars may be necessary, and laparoscopic surgery has recently been used for gastric bezoar removal.7,11 Endoscopic options that have been attempted include endoscopic suction and mechanical fragmentation of the bezoar using a pulsating jet of water with subsequent extraction.8,9
Coca-Cola has recently been used to dissolve phytobezoars with administration via nasogastric lavage, intrabezoar injection during endoscopy, ingestion, or a combination of these approaches.3,12-16
This case series documents the successful treatment of phytobezoars in 3 patients using ingestion of
Diet Coke and cellulase for 1–2 months. This case series is the first to report the combined use of cellulase and Diet Coke for phytobezoar dissolution. The simple ingestion of Diet Coke eliminated the need for nasogastric tube placement or complicated endoscopic procedures in these patients.
As cellulose is a major component of phytobezoars, it is logical that the enzyme cellulase could help to dissolve the concretions. The specific mechanism of action of cellulase is thought to consist of an attack on the leucoanthocyanidin-hemicellulose-cellulose bonds of the bezoar, resulting in the bezoar’s dissolution.1 As a pure form of cellulase is not available by prescription in the United States, the patients in this case series were told to obtain an oral enzymatic supplement that contained the largest amount of cellulase that they could find. The first 2 patients used Enzymedica Digest Gold (Enzymedica), which contains 3,000 units of cellulase per capsule. This preparation also contains glucoamylase, alpha-galactosidase, phytase/pectinase, xylanase, hemicellulase, and beta-glucanase. As phytobezoars may be composed of fruit and vegetable materials other than cellulose, it is reasonable to speculate that the other enzymes (eg, hemicellulase) in these capsules may have contributed to the dissolution of the bezoars. Unfortunately, we were unable to identify the exact brand of the cellulase supplement used by the third patient.
The mechanism of action of Coca-Cola for the dissolution of phytobezoars is less certain. It is believed that the sodium bicarbonate found in Coca-Cola has a mucolytic effect, and the penetration of carbon dioxide bubbles into the bezoar is thought to digest the fibers of the concretion.14 Additionally, Coca-Cola has a pH of 2.6 and contains both phosphoric and carbonic acids. This pH is close to that of normal gastric secretions (pH of 1–2). Since acid is important for digesting fiber, it has been suggested that
Coca-Cola exerts its effect by acidifying gastric contents.3 This mechanism of action is consistent with the fact that low gastric acidity is associated with phytobezoar formation.4 Diet Coke would presumably have the same mechanism of action because it is also made from carbonated water and phosphoric acid. It appears that substituting
Diet Coke for Coca-Cola did not adversely affect bezoar dissolution; however, this observation is complicated by the fact that cellulase supplements were also used in this case series. Diet Coke is preferable to nondiet Coca-Cola, as many patients with bezoars have underlying diabetes mellitus and, thus, need to limit intake of dietary sugar. It is unknown to what extent the positive results of this case series could be attributed to the use of Diet Coke, cellulase, or other enzymes in the cellulase supplement. Other case reports have shown similar success rates for the dissolution of bezoars with cellulase ingestion, and Coca-Cola ingestion has been successful for bezoar dissolution in a case report.5,6,14
A limitation of this treatment approach is the lack of readily available, standardized-dose cellulase capsules without other digestive enzymes. To fully elucidate whether any of the other enzymes in the supplement contributed toward bezoar resolution in this case series, it would be necessary to use a pure form of cellulase in a standard dose. Unfortunately, such a preparation is not commercially available at the present time. Another limitation of this case series is that 1 patient was also treated with onabotulinumtoxin A, which is a potential confounding variable.
Additionally, it is important to determine whether both cellulase and Diet Coke need to be used together, as it is possible that equally successful results could occur with only 1 of the 2 agents. In previous case reports, successful phytobezoar dissolution has been reported with cellulase or Coca-Cola ingestion alone in a very small number of patients.
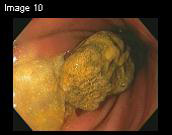
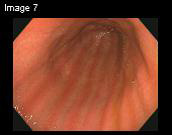
Figure 1. An endoscopic view of the gastric phytobezoar in
Patient #3 before treatment (A). After treatment, there was
complete resolution of the phytobezoar (B).
Table 1. Summary of Patient Characteristics
| Age (years) |
Gender | Risk factors | Presenting symptoms | Length of treatment (weeks) |
|
| Patient #1* | 68 | Male | Diabetic gastroparesis and prior esophagectomy with gastric pull-up |
Postprandial bloating, nausea, and vomiting |
8 |
| Patient #2 | 76 | Male | Diabetes mellitus | Postprandial dysphagia and belching |
6 |
| Patient #3 | 83 | Female | Billroth I gastrectomy | Postprandial nausea and vomiting, decreased appetite, and weight loss |
8 |
*The patient also received onabotulinumtoxin A (Botox, Allergan) injections to the pylorus for treatment of diabetic gastroparesis.
Summary
The findings of this case series support the combined use of cellulase and Diet Coke as a simple, noninvasive, and effective treatment for gastric phytobezoars. Future evaluation is necessary to assess and compare the efficacy of each agent as monotherapy as well as to evaluate the combination therapy in more patients.
The authors have no conflicts of interest to report.
References
1. Walker-Renard P. Update on the medicinal management of phytobezoars. Am J Gastroenterol. 1993;88:1663-1666.
2. Andrus CH, Ponsky JL. Bezoars: classification, pathophysiology, and treatment. Am J Gastroenterol. 1988;83:476-478.
3. Ladas SD, Triantafyllou K, Tzathas C, Tassios P, Rokkas T, Raptis SA. Gastric phytobezoars may be treated by nasogastric coca-cola lavage. Eur J Gastroenterol Hepatol. 2002;14:801-803.
4. Rider JA, Foresti-Lorente RF, Garrido J, et al. Gastric bezoars: treatment and prevention. Am J Gastroenterol. 1984;79:357-359.
5. Pollard HB, Block GE. Rapid dissolution of phytobezoar by cellulase enzyme. Am J Surg. 1968;116:933-936.
6. Smith BH, Mollot M, Berk JE. Use of cellulase for phytobezoar dissolution. Am J Gastroenterol. 1980;73:257-259.
7. Krausz MM, Moriel EZ, Ayalon A, Pode D, Durst AL. Surgical aspects of gastrointestinal persimmon phytobezoar treatment. Am J Surg. 1986;152:526-530.
8. Madsen R, Skibba RM, Galvan A, Striplin C, Scott P. Gastric bezoars: a technique of endoscopic removal. Am J Dig Dis. 1978;23:717-719.
9. Blam ME, Lichtenstein GR. A new endoscopic technique for the removal of gastric phytobezoars. Gastrointest Endosc. 2000;52:404-408.
10. Gayà J, Barranco L, Llompart A, Reyes J, Obrador A. Persimmon bezoars: a successful combined therapy. Gastrointest Endosc. 2002;55:581-583.
11. Song KY, Choi BJ, Kim SN, Park CH. Laparoscopic removal of gastric bezoar. Surg Laparosc Endosc Percutan Tech. 2007;17:42-44.
12. Kato H, Nakamura M, Orito E, Ueda R, Mizokami M. The first report of successful nasogastric coca-cola lavage treatment for bitter persimmon phytobezoars in Japan. Am J Gastroenterol. 2003;98:1662-1663.
13. Sechopoulos P, Robotis JF, Rokkas T. Gastric bezoar treated endoscopically with a carbonated beverage: case report. Gastrointest Endosc. 2004;60:662-664.
14. Okamoto Y, Yamauchi M, Sugihara K, Kato H, Nagao M. Is coca-cola effective for dissolving phytobezoars? Eur J Gastroenterol Hepatol. 2007;19:611-612.
15. Chung YW, Han DS, Park YK, et al. Huge gastric diospyrobezoars successfully treated by oral intake and endoscopic injection of coca-cola. Dig Liver Dis. 2006;38:515-517.
16. Harikumar R, Kunnel P, Sunilraj R. Dissolution of pharmacobezoar using carbonated beverage. Indian J Gastroenterol. 2008;27:245-246.
REVIEW
Gastrointestinal Bezoars: History and Current Treatment Paradigms
Katharine Eng, MD
Marsha Kay, MD
Department of Pediatric Gastroenterology and Nutrition, Children’s Hospital, Cleveland Clinic Foundation,
Cleveland, Ohio
Address correspondence to:
Dr. Katharine Eng, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44106; Tel: 216-444-3564; E-mail: engk2@ccf.org
Gastrointestinal (GI) bezoars are aggregates of inedible or undigested material found in the GI tract. For many centuries, bezoars have been found in the digestive tracts of both humans and animals, and although bezoars are most commonly found in the stomach, they can be found anywhere in the GI tract.1 The term “bezoar” is thought to be derived from the Arabic word “badzehr” or the Persian word “panzehr,” both of which mean “counterpoison” or “antidote.” In ancient times, bezoars from animals were thought to have medicinal and magical properties, and they were considered antidotes to a variety of poisons and diseases. Bezoars were introduced to Europe from the Middle East during the 11th century, and they were popular as medicinal remedies; however, their use started to fall out of favor by the 18th century.2 In the 1500s, the famous surgeon Ambroise Paré tested the healing properties of a bezoar stone. A cook in the king’s court had been caught stealing fine silver and was sentenced to death by hanging. As an alternative, the cook was granted the opportunity to receive a poison followed by a bezoar as a potential antidote under the supervision of Paré. It was agreed that if the cook survived the poison, his life would be spared. The cook lived for only 7 hours; thus, Paré concluded that the bezoar stone could not cure all poisons.3 Currently, the term “bezoar” is not used to refer to an unsuccessful antidote but rather to a potentially serious medical problem that requires timely diagnosis and appropriate therapy.
The formation of bezoars can occur in individuals with normal GI physiology and anatomy. However, patients with altered GI anatomy and/or motility are at an increased risk for the development of bezoars. Risk factors for bezoar formation include a partial gastrectomy with or without a vagotomy, diabetes mellitus complicated by gastroparesis, or other systemic illnesses that may affect GI motility.4,5 Other predisposing factors include poor mastication, excessive intake of fiber, cystic fibrosis, or psychiatric illness.1,6,7
For successful management of bezoars, it is important to distinguish among their various types. Bezoars are typically grouped into 1 of 4 types according to their composition: phytobezoars (which are composed of indigestible food particles that are found in vegetable or fruit fibers), trichobezoars (which are composed of a conglomeration of hair and food particles), lactobezoars (which are composed of milk protein), or pharmacobezoars (which are concretions of various medications).6 Phytobezoars are the most common type of bezoars, accounting for approximately 40% of all reported bezoars. Diospyrobezoars, a subset of phytobezoars, are composed of persimmons and can be particularly hard in consistency, as they are formed by the agglutination of tannins in fruit skins.1
The initial presentation of a bezoar often depends on its composition. Lactobezoars may present in premature infants or newborns with symptoms of feeding intolerance, abdominal distension, irritability, and/or vomiting. A physical examination may reveal a palpable abdominal mass in these patients.8 Pharmacobezoars can present with symptoms of gastric outlet obstruction, but these bezoars can also produce symptoms due to their pharmacologic properties. As a result, there is an increased potential for drug intoxication in these patients.9 Trichobezoars can take time to form—sometimes up to several years—and these bezoars may first present with subtle symptoms such as nausea or early satiety. However, as trichobezoars grow in size, they may present with epigastric pain, gastric outlet obstruction, ulceration, GI bleeding, and/or,
potentially, perforation.6,10 Phytobezoars usually form more rapidly than trichobezoars. Phytobezoars can present with nausea, vomiting, and/or symptoms of gastric outlet obstruction. These symptoms are similar to those reported in the 3 patients described by Kramer and Pochapin.11 Complications from phytobezoars can include ulceration, bleeding, bowel obstruction, and/or perforation. Therefore, appropriate and prompt diagnosis is important in the care of patients with bezoars in order to prevent the development of potentially severe complications.
The diagnosis of a bezoar involves obtaining a thorough patient history, which includes screening patients for risk factors and questioning patients regarding their diet and medications. A physical examination may occasionally reveal a palpable abdominal mass. Halitosis may signify the presence of putrefying material in the stomach, and clinicians may observe patches of alopecia in patients with trichobezoars similar to those seen in individuals with trichotillomania (an impulse control disorder involving a compulsive urge to pull out one’s hair).
In terms of imaging, plain abdominal radiographs are often the initial modality for diagnosing bezoars. In 1 study, an abdominal radiograph raised suspicion for a bezoar in 56% of cases.12 Barium studies are also useful for identifying bezoars and estimating their size. However, barium studies can interfere with diagnostic and therapeutic endoscopic procedures by impeding visualization; this consequence should be carefully considered before the administration of oral contrast. Both ultrasound and computed tomography (CT) scans have been found to be reliable methods for diagnosing GI bezoars, although CT scans are more accurate and can more readily identify the presence of any additional GI bezoars that may be present.13 However, endoscopy remains the diagnostic method of choice for gastric bezoars because it enables visualization and tissue sampling of the bezoar; in addition, endoscopy can occasionally have therapeutic applications.14 Kramer and Pochapin used endoscopy to diagnose a phytobezoar in each of their patients.11
The goals of bezoar treatment are the removal of the bezoar and the prevention of bezoar recurrence. Knowing the type and location of the bezoar are important for determining appropriate management. Management strategies for gastric phytobezoars can be divided into 3 categories: lavage or dissolution, fragmentation, and/or retrieval. There are several methods for dissolving and/or retrieving bezoars, and these methods may be used in conjunction, depending on the type and location of the bezoar.1 As discussed by Kramer and Pochapin, phytobezoars were often treated surgically prior to the 1960s.11 Since then, a wider range of therapeutic options have been used, including acetylcysteine, papain, metoclopramide, cellulase, and instillation of Coca-Cola (The Coca-Cola Company), the latter of which was first reported in 2002.15-19 However, some of these agents are associated with adverse reactions; for example, case reports have found gastric ulceration, esophageal perforation, and hypernatremia with the use of papain, which is a proteolytic enzyme.20 Both cellulase and Coca-Cola have been better tolerated, without any adverse effects reported to date.19-24 A recent paper reviewing the medical management of bezoars reported on the administration of 3–5 g of cellulase enzyme that was dissolved in 300–500 mL of water and administered orally each day for 2–5 days.6 Coca-Cola administration has been performed by nasogastric lavage (NG) of 3 L of Coca-Cola over 12 hours, oral ingestion, as well as endoscopic injection and irrigation.19,23-25
Kramer and Pochapin reported the concomitant use of Diet Coke (The Coca-Cola Company) and cellulase ingestion for the dissolution of gastric phytobezoars in 3 patients.11 The patients were instructed to drink one 12-oz can of Diet Coke twice daily and to take
1 cellulase tablet twice daily until resolution of the bezoar could be endoscopically confirmed, a process that took
6–8 weeks.11 This case series is the first to report the combined use of cellulase and Diet Coke ingestion for dissolution of phytobezoars. Prior studies have examined the use of either cellulase or Coca-Cola over a shorter period of time. In particular, Coca-Cola has often been administered via NG lavage over 12 hours, a process that requires hospitalization. The treatment approach reported by Kramer and Pochapin is interesting, as it has the potential to eliminate the need for inpatient hospitalization and NG tube placement, thus increasing patient comfort.11 It is unclear how many endoscopic sessions are needed with this treatment approach; if the number of sessions is minimal, this approach may also be a less expensive option for treatment of gastric phytobezoars, as it avoids the cost of hospitalization. Another novel aspect of this case series is that it is one of the first studies to show longer-term use of cellulase without any adverse effects; most studies have reported the use of this agent for only 2–5 days.
In addition to the dissolution therapy described above, there are a variety of methods that can be used for the fragmentation and retrieval of bezoars. Endoscopic therapy has focused on mechanical disruption via a variety of instruments, including polypectomy snares, tripod forceps, water piks, neodymium yttrium aluminum garnet lasers, and bezotriptors (modified lithotripters that break up bezoars with shock waves).26,27 A new endoscopic technique involves the removal of gastric phytobezoars via suction through a large-channel endoscope. In a case series, this technique was found to be a safe, effective, and rapid method of removing large gastric phytobezoars.28 Although endoscopic therapy may be the procedure of choice in terms of fragmentation and retrieval, surgical removal should be considered in patients who fail medical therapy or who have complications such as significant bleeding, obstruction, and/or perforation.
Other types of bezoars require different management strategies, which will be briefly outlined here. Unlike phytobezoars, trichobezoars are often resistant to enzymatic dissolution; therefore, most trichobezoars are surgically removed, although endoscopic removal has been successful in some cases.6 On the other hand, lactobezoars are typically managed conservatively, with the most common treatment approach involving the patient being nil per mouth and receiving intravenous fluids alone or in combination with gastric lavage; however, endoscopy and surgery are occasionally needed.8 The treatment of pharmacobezoars depends on the pharmaceutical agent and the patient’s clinical status; treatment options can be quite varied and can range from gastric decontamination to dissolution therapy, bowel irrigation, or more aggressive interventions such as endoscopic or surgical removal.9
Summary
As GI bezoars are a potentially serious problem, it is important to be aware of their risk factors, as well as subtle clinical findings that may facilitate early investigation and diagnosis. Knowing the location and type of the bezoar are important, as this information influences patient management. Treatment of gastric phytobezoars has included lavage and dissolution therapy with a variety of dissolution products—including a novel and noninvasive combination technique reported by Kramer and Pochapin—as well as endoscopic fragmentation and retrieval, and surgery.11 Following removal of a bezoar, it is prudent to prevent future occurrences via dietary counseling, avoidance of certain medications, and correction of underlying motility problems if present.
The authors have no conflicts of interest to report.
References
1. Kuhn BR, Mezoff AG. Bezoars. In: Wyllie R, Hyams JS, Kay M, eds. Pediatric Gastrointestinal and Liver Disease. 4th ed. Philadelphia, Pennsylvania: Elsevier Saunders; 2011:319-322.
2. Williams RS. The fascinating history of bezoars. Med J Aust. 1986;145:613-614.
3. Paget S. Ambroise Paré and His Times, 1510–1590. London, United Kingdom: G. P. Putnam Son’s; 1897:186-187.
4. Bowden TA Jr, Hooks VH 3rd, Mansberger AR Jr. The stomach after surgery: an endoscopic perspective. Ann Surg. 1983;197:637-644.
5. Hewitt AN, Levine MS, Rubesin SE, Laufer I. Gastric bezoars: reassessment of clinical and radiographic findings in 19 patients. Br J Radiol. 2009;82:901-907.
6. Sanders M. Bezoars: from mystical charms to medical and nutritional management. Practical Gastroenterology. 2004;28:37-50.
7. Kement M, Ozlem N, Colak E, Kesmer S, Gezen C, Vural S. Synergistic effect of multiple predisposing risk factors on the development of bezoars. World J Gastroenterol. 2012;18:960-964.
8. Heinz-Erian P, Gassner I, Klein-Franke A, et al. Gastric lactobezoar—a rare disorder? Orphanet J Rare Dis. 2012;7:3.
9. Simpson SE. Pharmacobezoars described and demystified. Clin Toxicol (Phila). 2011;49:72-89.
10. Ciampa A, Moore BE, Listerud RG, Kydd D, Kim RD. Giant trichophytobezoar in a pediatric patient with trichotillomania. Pediatr Radiol. 2003;33:219-220.
11. Kramer SJ, Pochapin MB. Gastric phytobezoar dissolution with ingestion of diet coke and cellulase. Gastroenterol Hepatol (N Y). 2012;8:770-772.
12. Erzurumlu K, Malazgirt Z, Bektas A, et al. Gastrointestinal bezoars: a retrospective analysis of 34 cases. World J Gastroenterol. 2005;11:1813-1817.
13. Ripolles T, Garcia-Aguayo J, Martinez MJ, Gil P. Gastrointestinal bezoars: sonographic and CT characteristics. AJR Am J Roentgenol. 2001;177:65-69.
14. Wang YG, Seitz U, Li ZL, Soehendra N, Qiao XA. Endoscopic management of huge bezoars. Endoscopy. 1998;30:371-374.
15. Schlang HA. Acetylcysteine in removal of bezoar. JAMA. 1970;214:1329.
16. Walker-Renard P. Update on the medicinal management of phytobezoars. Am J Gastroenterol. 1993;88:1663-1666.
17. Pollard HB, Block GE. Rapid dissolution of phytobezoar by cellulase enzyme. Am J Surg. 1968;116:933-936.
18. Holloway WD, Lee SP, Nicholson GI. The composition and dissolution of phytobezoars. Arch Pathol Lab Med. 1980;104:159-161.
19. Ladas SD, Triantafyllou K, Tzathas C, Tassios P, Rokkas T, Raptis SA. Gastric phytobezoars may be treated by nasogastric coca-cola lavage. Eur J Gastroenterol Hepatol. 2002;14:801-803.
20. Zarling EJ, Moeller DD. Bezoar therapy: complication using Adolph’s meat tenderizer and alternatives from literature review. Arch Intern Med. 1981;141:1669-1670.
21. Lee SP, Holloway WD, Nicholson GI. The medical dissolution of phytobezoars using cellulase. Br J Surg. 1977;64:403-405.
22. Zarling EJ, Thompson LE. Nonpersimmon gastric phytobezoar: a benign recurrent condition. Arch Intern Med. 1984;144:959-961.
23. Okamoto Y, Yamauchi M, Sugihara K, Kato H, Nagao M. Is coca-cola effective for dissolving phytobezoars? Eur J Gastroenterol Hepatol. 2007;19:611-612.
24. Lin CS, Tung CF, Peng YC, Chow WK, Chang CS, Hu WH. Successful treatment with a combination of endoscopic injection and irrigation with coca cola for gastric bezoar–induced gastric outlet obstruction. J Chin Med Assoc. 2008;71:49-52.
25. Chung YW, Han DS, Park YK, et al. Huge gastric diospyrobezoars successfully treated by oral intake and endoscopic injection of coca-cola. Dig Liver Dis. 2006;38:515-517.
26. Naveau S, Poynard T, Zourabichvili O, Poitrine A, Chaput JC. Gastric phytobezoar destruction by Nd:YAG laser therapy. Gastrointest Endosc. 1986;32:430-431.
27. Benes J, Chmel J, Jodl J, Stuka C, Nevoral J. Treatment of a gastric bezoar by extracorporeal shock wave lithotripsy. Endoscopy. 1991;23:346-348.
28. Blam ME, Lichtenstein GR. A new endoscopic technique for the removal of gastric phytobezoars. Gastrointest Endosc. 2000;52:404-408.
